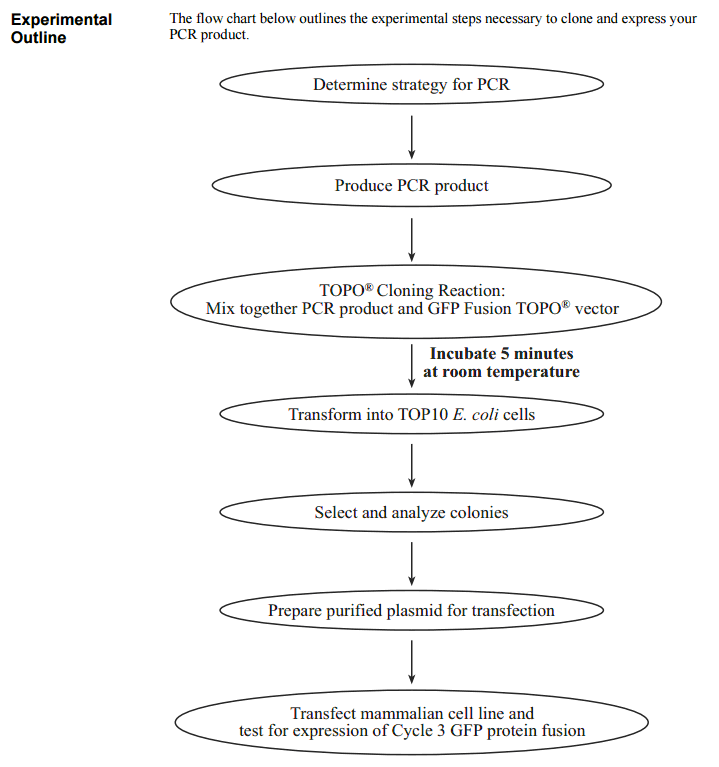
Life Technologies' CT GFP-fusion TOPO Expression kit provides pcDNA3.1CT-GFP-TOPO vector for highly efficient, 5-minute cloning that fuses the green fluorescent protein (GFP) to the C-terminal end your Taq polymerase-amplified gene product. The pcDNA3.1 vector delivers high-level expression of transient or stable GFP fusion proteins in a wide range of mammalian cells Expression is easily detected in living cells using fluorescence. In addition, recombinant proteins expressed from these vectors can be detected on western blots using GFP Antiserum.
GFP Fusion TOPO Cloning provides a highly efficient, 5-minute, one-step cloning strategy ("TOPO Cloning") for the direct fusion of Taq polymerase-amplified PCR products to the green fluorescent protein (GFP). No ligase, post-PCR procedures, or PCR primers containing specific sequences are required. Once cloned, analyzed, and transfected, the GFP fusion protein will express directly in mammalian cell lines. Two kits are available that allow you to create N-terminal (NT-GFP Fusion TOPO TA Expression Kit) or C-terminal (CT-GFP Fusion TOPO TA Expression Kit) GFP fusions. 使用方法 The plasmid vectors (pcDNA3.1/CT-GFP-TOPO or pcDNA3.1/NT-GFP-TOPO) are supplied linearized with:
Single 3′ thymidine (T) overhangs for TA Cloning
Topoisomerase covalently bound to the vector (referred to as "activated" vector) Taq polymerase has a nontemplate-dependent terminal transferase activity that adds a single deoxyadenosine (A) to the 3′ ends of PCR products. The linearized vector supplied in this kit has single, overhanging 3′ deoxythymidine (T) residues. This allows PCR products to ligate efficiently with the vector.
Topoisomerase I from Vaccinia virus binds to duplex DNA at specific sites and cleaves the phosphodiester backbone after 5′-CCCTT in one strand (Shuman, 1991). The energy from the broken phosphodiester backbone is conserved by formation of a covalent bond between the 3′ phosphate of the cleaved strand and a tyrosyl residue (Tyr-274) of topoisomerase I. The phospho-tyrosyl bond between the DNA and enzyme can subsequently be attacked by the 5′ hydroxyl of the original cleaved strand, reversing the reaction and releasing topoisomerase (Shuman, 1994). TOPO Cloning exploits this reaction to efficiently clone PCR products (see below) 绿色荧光蛋白GFP The GFP gene used in these vectors is described in Crameri et al., 1996. In this paper, the codon usage was optimized for expression in E. coli and three cycles of DNA shuffling were used to generate a mutant form of GFP that expressed well in mammalian cells and has the following characteristics:
Excitation and emission maxima that are the same as wild-type GFP (395 nm and 478 nm for primary and secondary excitation, respectively, and 507 nm for emission)
High solubility in E. coli for visual detection of transformed cells (if expressed from a promoter recognized by E. coli. Please note that there is no bacterial promoter upstream of the multiple cloning site in the GFP Fusion TOPO vectors.)
>40-fold increase in fluorescent yield over wild-type GFP
This GFP protein will be subsequently referred to as Cycle 3 GFP to differentiate it from wild-type GFP.