pVPack逆病毒包装载体描述 Choice of env-Expressing Vector
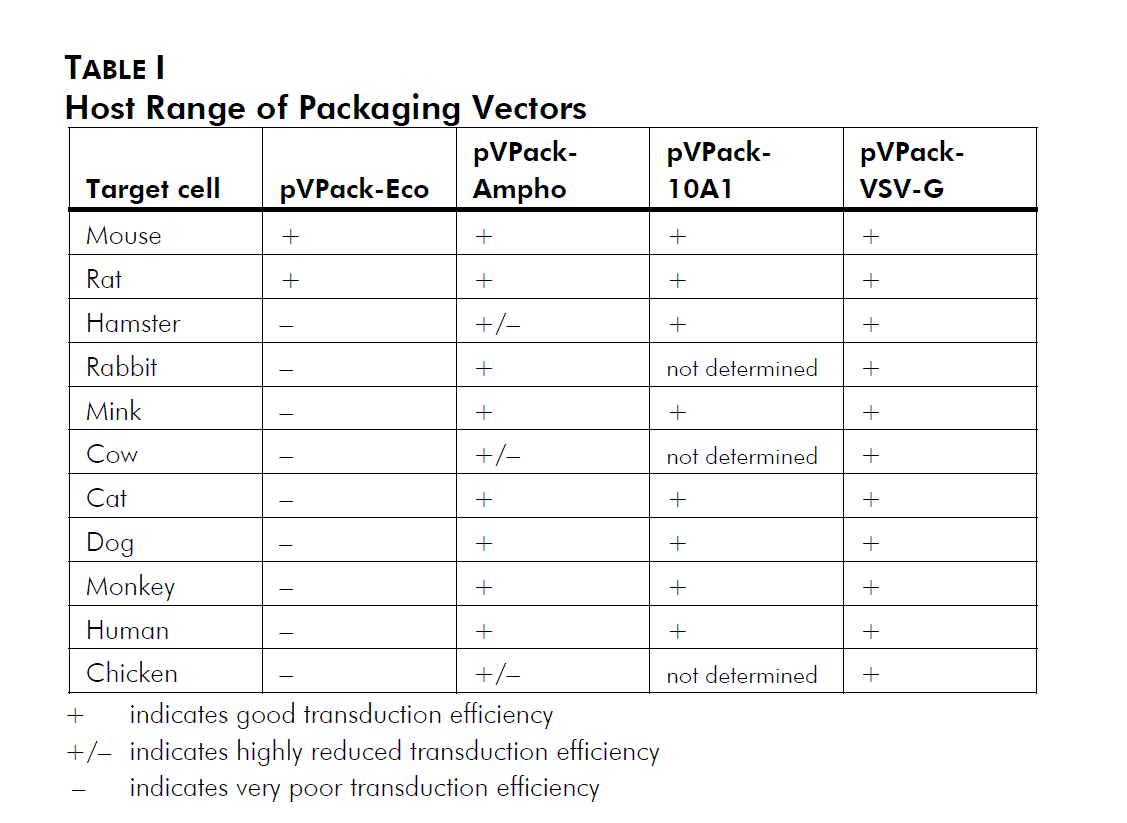
In addition to the gag-pol expression vector pVPack-GP, the pVPack vector system offers 4 different env-expressing vectors. Which of those 4 is selected depends on the choice of host cell type; see Table I and Miller (1997).2 The pVPack-Eco vector is the safest vector, providing experiments can be performed in transduced mouse or rat cells; ecotropic virus infects human cells with extremely low efficiency. The amphotropic envelope protein has historically been the protein of choice for infection of human and other mammalian cell lines. More recently the 10A1 envelope protein has been used due to its increased versatility relative to the amphotropic protein. The 10A1 protein recognizes the same cell-surface receptor as the amphotropic envelope protein plus a second receptor, and thus can essentially infect any cell that an amphotropic virus can infect, although in some cases with a higher efficiency. The ecotropic, amphotropic and 10A1 proteins are all natural MMLV variants, and are all relatively labile and thus considered relatively safe compared with other viral systems. The vesicular stomatitis virus G protein (VSV-G) is rapidly becoming the most popular envelope protein. Unlike the other three MMLV-derived envelope proteins which recognize cell surface receptors, VSV-G recognizes a phospholipid that is present on all cell types, and thus can theoretically allow the efficient infection of any mitotic cell.3 Special precautions must be used when working with this vector (see Preprotocol Safety Considerations).
Vector Features
Figures 2 and 3 illustrates the important features of the vectors in the pVPack system. The expression of both the gag-pol elements in the pVPack-GP vector and the envelope elements in the env-expressing vectors are driven by the CMV promoter. Each of these vectors also contains an internal ribosome entry site (IRES) linked to a downstream drug-resistance cassette that enables the selection of stable producer lines. The vector pVPack-GP and the env-expressing vectors employ different resistance cassettes, hisD and puromycin, respectively. Methods for selecting stable producer lines are discussed in Hartman and Mulligan (1988)4 and Wirth and colleagues (1988).5
Notes
If a compatible MMLV-based retroviral vector is chosen, all three vectors can be maintained simultaneously. Although stable VSV-G-expressing cells lines have been successfully constructed, in general they are of poor quality due to the toxicity of VSV-G. For the production of VSV-G pseudotyped virus, transient transfection rather than selection of stable cell lines is recommended.
The bacterial origin of replication, pUC, and ampicillin resistance cassette are included to permit maintenance and production of the vectors in E. coli.
Choice of Expression Vector
Any MMLV-based retroviral vector for gene delivery and expression can be used with the pVPack vector system to produce high-titer retroviral stocks. The Stratagene pFB and pCFB retroviral vectors and ViraPort retroviral cDNA libraries are compatible with the Stratagene pVPack system. They contain the elements necessary for virion packaging: a bacterial origin of replication and ampicillin-resistance gene from pBR322, an extended MMLV packaging signal (ψ+), and a multiple cloning site (MCS) that is located between the MMLV 5′ and 3′ long terminal repeat sequences (LTRs).
pFB-Neo-LacZ, pFB-hrGFP, and pFB-Luc Control Vectors
The pFB-Neo-LacZ plasmid vector provided with the kit contains a bicistronic transcript; the β-galactosidase gene is expressed from the first open reading frame, and is followed by the neomycin-resistance marker downstream from an IRES. The vector may be used as an expression control, and can also be used to determine viral titer by FACS, in situ staining with X-gal, or G418-resistant colony formation.
Also available for use as a positive control is the vector pFB-hrGFP (Stratagene Catalog #240027), which contains coding sequence for the humanized green fluorescent protein from a novel marine organism. The hrGFP-expressing vector can be used to determine the transfection efficiency of the packaging cell line and to determine viral titer by FACS.
The pFB-Luc control* (also available separately) allows a qualitative assessment of the efficiency with which the target cell type is transduced by retrovirus. Direct comparisons between the cell lines based on luciferase activity should be made with caution however, as differences in luciferase activity may be due to cell type-dependent differences in luciferase expression rather than differences in transduction efficiencies.